
Lithium-Ion batteries, if subjected or used under stressed conditions namely high temperatures, overcharging, high current, over charging/discharging, high mechanical stress degrades Lithium Ion batteries, and may result in hazardous fire due to thermal runaway.
Temperature:LIBs if used under higher than rated temperature results in acceleration of chemical reactions in LIB resulting in substantial capacity loss and increase in internal resistance, and risk of thermal runaway.Discharging LIB above it's C rating is generally not recommended and can have a negative impact on the usable energy density. It's important to adhere to the manufacturer's recommended operating practices and discharge the battery at or below its rated C rate to ensure optimal performance and maximum usable energy density.
Discharging a battery above its C rating causes the battery to experience higher levels of stress and degrade more quickly than if it were discharged at or below its rated C rate it was designed for, resulting in......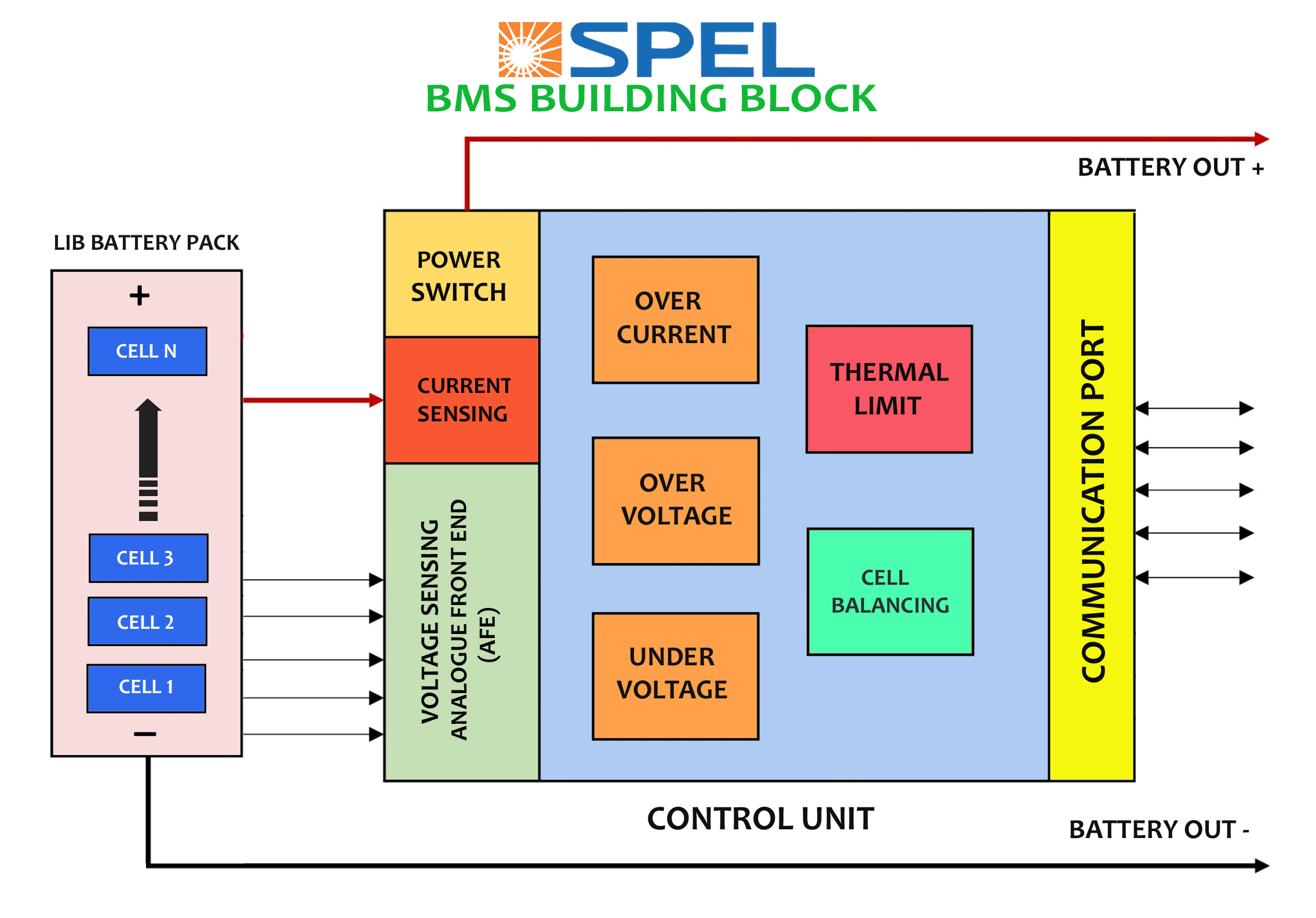
Battery Management System Building Blocks